X Chromosome Inactivation: Breakthroughs in Genetic Disorders
X chromosome inactivation is a fascinating biological phenomenon crucial for female mammals, ensuring that only one of the two X chromosomes present in females is active, while the other is silenced. This process plays a significant role in maintaining genetic balance and has profound implications in genetics research, particularly in understanding disorders like Fragile X Syndrome and Rett Syndrome, both linked to mutations on the X chromosome. Pioneering research led by Jeannie Lee at Harvard Medical School has shed light on the intricate mechanisms involved in this chromosomal silencing. By exploring how cells orchestrate X chromosome inactivation, we move closer to potentially curing genetic disorders that affect thousands worldwide. The ongoing studies into X inactivation not only enhance our understanding of genetics but may also pave the way for groundbreaking treatments for X-linked diseases.
The process of X chromosome inactivation, often referred to as Xi or chromosomal silencing, is essential for regulating gene expression in females. This intricate mechanism ensures that, despite having two copies of the X chromosome, females achieve dosage compensation similar to males, who possess only one. Understanding how this genetic system operates is pivotal for addressing conditions related to the X chromosome, such as Fragile X Disease and Rett Syndrome. Research initiatives undertaken by prominent scientists like Jeannie Lee have made significant strides in uncovering the factors that contribute to this phenomenon, illuminating potential pathways for therapeutic interventions. As the field progresses, the implications for genetic disorders become increasingly profound, suggesting that targeting these processes could lead to innovative treatments.
Understanding X Chromosome Inactivation
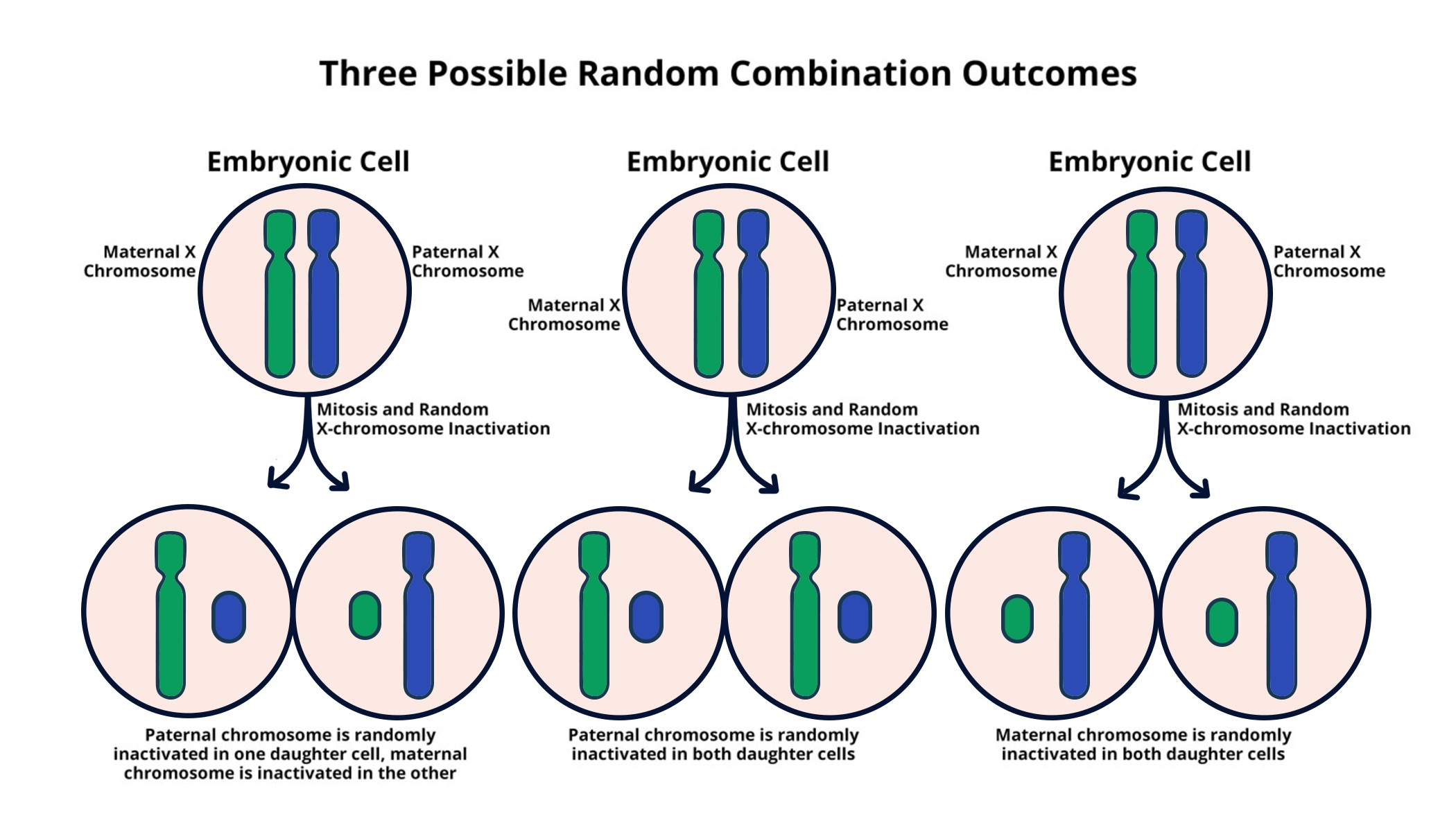
X chromosome inactivation (XCI) is a crucial biological process that occurs in female mammals to balance gene dosage between the sexes. In females, who possess two X chromosomes, one X is randomly inactivated in each cell, leading to the phenomenon where females are essentially mosaics of two cell populations, each expressing a different X chromosome. This intricate mechanism is vital for normal development and cellular function. Jeannie T. Lee’s research at Harvard emphasizes the importance of this process, revealing how it relies on a unique gelatinous substance that acts as a mediator for chromosomal silencing.
The process of XCI begins with the expression of the Xist gene, which produces an RNA molecule essential for coating and silencing one of the X chromosomes. Lee’s team has uncovered that Xist engages in a complex interaction with the gelatinous substance surrounding chromosomes, akin to Jell-O, which helps prevent chromosomes from getting entangled. This breakthrough enriches our understanding of X chromosome dynamics and opens up new avenues in genetics research, especially regarding the therapeutic implications for disorders linked to X chromosome mutations.
Impact of XCI on Genetic Disorders
Mutations on the X chromosome are responsible for several genetic disorders, including Fragile X Syndrome and Rett Syndrome, both of which have shown promise in potential treatment approaches derived from Lee’s research. By manipulating the mechanisms of XCI, especially the process by which Xist interacts with chromosomal structures, researchers can possibly reactivate silenced genes that carry beneficial functions for individuals affected by these syndromes. This represents a significant leap in the field of genetics, offering hope to countless individuals who have historically faced limited options for effective treatments.
Understanding how X chromosome inactivation affects gene expression can lead to innovative treatment strategies. For instance, by selectively unsilencing genes responsible for conditions like Fragile X Syndrome, it may be possible to restore normal function in affected individuals. Lee’s work indicates that this approach could not only preserve healthy gene function but also provide a targeted remedy for dysfunction arising from mutations without adverse effects on other functioning genes. This precision in medicine showcases a hopeful future stemming from foundational genetics research.
Jeannie Lee’s Breakthroughs in Chromosomal Silencing
Jeannie T. Lee’s groundbreaking research has transformed our understanding of chromosomal silencing, particularly regarding the X chromosome. By revealing how a substance akin to Jell-O contributes to X chromosome inactivation, she has opened up new possibilities for therapeutic interventions that could alleviate the impact of genetic disorders. The laboratory’s focus on manipulating the XCI process offers avenues that were previously only theoretical, bringing us closer to potential clinical applications. Her work emphasizes perseverance in scientific inquiry, as it took years of detailed study to uncover these mechanisms.
The therapeutic implications of Lee’s discoveries cannot be understated. With the ability to free inactivated X chromosomes, researchers could potentially cure diseases that arise from mutations on these chromosomes. The possibility of restoring function to genes that would otherwise remain inaccessible has significant ramifications for conditions that disproportionately affect females due to X chromosome heritage. Lee’s research underscores the intersection between fundamental genetics research and its real-world applications, a combination that may lead to revolutionary treatments for conditions such as Fragile X Syndrome and Rett Syndrome.
The Role of Chromosomal Gelatin in XCI
The unique gelatinous substance identified by Lee and her team serves as more than just a passive component of chromosomal structure; it plays an active role in mediating X chromosome inactivation. This Jell-O-like material creates an environment where genetic silencing can occur efficiently. By adjusting the physical properties of the gelatin when Xist is introduced, the inactivation process can unfold smoothly, allowing specific regions of the X chromosome to become inaccessible for transcription. Understanding this and how it correlates with conditions like Fragile X Syndrome enhances the mechanisms behind gene expression and suppression.
Furthermore, the capacity to manipulate this gelatinous material could yield significant advancements in genetics research. The elasticity and viscosity changes induced by Xist reveal insights into chromosomal organization and gene regulation, potentially informing strategies to address genetic disorders. This innovative perspective highlights the importance of structural biology in gene functioning, merging the disciplines of cell biology and genetics. It points towards a future where fine-tuning chromosomal environments could lead to breakthrough treatments for a range of conditions beyond those associated with just the X chromosome.
Future Prospects of XCI Research
The future of X chromosome inactivation research under the guidance of researchers like Jeannie Lee is filled with potential for discovering new therapies for genetically-driven disorders. As scientists continue to explore the mechanics behind XCI, they uncover new biological pathways that could be harnessed for therapeutic benefits. With the recent advances made in isolating and manipulating the processes involved in X chromosome silencing, there is optimism surrounding the development of clinical trials aimed at tackling conditions like Fragile X Syndrome and Rett Syndrome.
Moreover, this line of research raises important questions about the broader implications of genetic therapy. As the understanding of X chromosome inactivation deepens, it may also reveal mechanisms that can be applied to other chromosomal disorders. There is a significant opportunity to address not only inherited X-linked conditions but also to enhance gene therapy techniques for a variety of genetic disorders, by utilizing insights gained from XCI studies. The interdisciplinary nature of this research, combining genetics, molecular biology, and clinical applications, reflects the evolving landscape of therapeutic development.
XCI: Bridging Basic Science and Clinical Application
The intersection of basic scientific research and clinical application is vividly illustrated in Jeannie Lee’s discoveries surrounding X chromosome inactivation. For over 25 years, her work laid the groundwork to decipher how XCI occurs and the implications of chromosomal silencing on genetic disorders. The pathway from understanding fundamental biological processes to developing therapeutics exemplifies the aim of translational research, where knowledge gained from laboratory experiments ultimately seeks to improve patient care and outcomes.
By focusing on the genetic mechanisms underlying diseases like Fragile X Syndrome and Rett Syndrome, researchers can design targeted therapies that not only aim to correct the genetic malfunction but also minimize the risk of unintended consequences on healthy gene function. The promise shown by Lee’s achievements inspires further exploration into the molecular workings of the X chromosome and how we might leverage these insights for the benefit of those affected by genetic disorders, representing a critical shift towards more personalized and effective treatment strategies in medicine.
The Link Between XCI and Genetic Therapy
The connection between X chromosome inactivation (XCI) and genetic therapies is an area of active exploration following breakthroughs made by researchers like Jeannie Lee. By understanding how XCI operates, especially in females who have two X chromosomes, scientists can devise techniques to reactivate silenced genes linked to developmental and cognitive disorders. Solving the mysteries of chromosomal silencing has the potential to unlock new strategies for genetic therapy that can provide hope for patients suffering from conditions like Fragile X Syndrome.
Furthermore, as investigations into XCI advance, they may lead to the development of methods to address both X-linked disorders and potentially other genetic conditions that involve similar mechanisms of gene regulation. This is a key area of focus given the increasing interest in gene therapy and precision medicine. Enhanced knowledge from studies on XCI can provide essential insights into how to decode complex genetic interactions and develop viable treatments that target specific genetic abnormalities. These findings contribute to a wider revolution in genetic healthcare that aims to improve outcomes for individuals across various genetic backgrounds.
The Future of Genetics Research Inspired by XCI
The advancements in understanding X chromosome inactivation (XCI) fosters a bright future for genetics research, inspiring new methodologies and therapeutic approaches that can impact numerous genetic disorders. The potential to unsilence inactivated genes opens pathways not only to treat Fragile X Syndrome and Rett Syndrome but could also lead to broader applications in other genetic conditions linked to chromosomal abnormalities. The ongoing research initiated by scientists like Jeannie Lee encourages collaboration and expansion of study into how chromosomal mechanisms can be utilized to improve health outcomes.
As genetic therapy continues to evolve, insights on XCI will be fundamental in shaping the next steps for clinical applications. Researchers may find novel ways to engage with chromosomal silencing and gene expression, ultimately driving research that targets both genetic and epigenetic factors in disorders. These explorations represent an exciting trajectory towards reshaping our understanding of genetics and the delivery of effective treatments, reaffirming the vital role that chromosomal studies play in unraveling the complexities of human health.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in genetics research?
X chromosome inactivation is a process where one of the two X chromosomes in females is silenced to ensure equal gene dosage in males and females. This is crucial in genetics research as it helps understand sex-linked disorders, such as Fragile X Syndrome and Rett Syndrome, by providing insights into chromosomal silencing mechanisms and potential therapeutic approaches.
How does X chromosome inactivation relate to Fragile X Syndrome and Rett Syndrome?
X chromosome inactivation is directly linked to Fragile X Syndrome and Rett Syndrome because mutations causing these disorders are often found on the X chromosome. Understanding how X chromosome inactivation works can lead to potential treatments by unsilencing the healthy gene version that is otherwise inactive, which is essential for alleviating symptoms of these syndromes.
What role does the Xist gene play in X chromosome inactivation?
The Xist gene produces an RNA molecule that plays a pivotal role in X chromosome inactivation by binding to the X chromosome and altering the surrounding chromosomal environment, or ‘Jell-O.’ This process initiates chromosomal silencing, making the X chromosome inactive and helping to balance gene expression between males and females.
How does Jeannie Lee’s research contribute to a better understanding of X chromosome inactivation?
Jeannie Lee’s research provides significant insights into the mechanisms of X chromosome inactivation, revealing how the Xist RNA interacts with the chromosomal structure. Her findings elucidate how chromosomal silencing occurs, which may lead to innovative treatments for X-linked disorders like Fragile X Syndrome and Rett Syndrome through potential methods to reactivate genes.
What are the potential therapeutic implications of X chromosome inactivation research?
The therapeutic implications of research on X chromosome inactivation are promising. By developing strategies to unsilence the X-linked genes associated with disorders like Fragile X Syndrome and Rett Syndrome, researchers aim to restore the function of mutated genes, offering hope for effective treatments with minimal side effects.
What challenges are faced in the clinical application of findings related to X chromosome inactivation?
One challenge in the clinical application of findings related to X chromosome inactivation is ensuring the safety and efficacy of therapies that reactivate silenced genes. Researchers are still investigating how to minimize side effects and understand why only mutated genes are affected while healthy genes remain largely unchanged during the therapeutic process.
Is X chromosome inactivation relevant to males with X-linked disorders?
Yes, X chromosome inactivation is relevant to males with X-linked disorders. While males typically do not undergo X inactivation, they can still possess mutations on their single X chromosome. Understanding how to manipulate X chromosome silencing could lead to therapies that also benefit males by addressing the issues caused by these mutations.
How has research on X chromosome inactivation evolved over the years?
Research on X chromosome inactivation has evolved significantly over the years, transitioning from fundamental studies on the process to potential therapeutic applications. Initially supported by years of National Institutes of Health funding, recent breakthroughs paved the way for understanding how X inactivation mechanisms can be leveraged for treating chromosomal disorders like Fragile X Syndrome and Rett Syndrome.
| Key Points |
|---|
| Females have two X chromosomes while males have one, leading to the need for one X chromosome to be inactivated in females. |
| This process of X chromosome inactivation has been a challenging topic in cell biology. |
| Jeannie Lee’s lab has made significant contributions to understanding how X chromosome inactivation occurs. |
| The inactivation process involves a gelatinous substance around chromosomes that changes properties based on interactions with RNA molecule Xist. |
| Xist encircles the X chromosome, altering the biophysical properties of the surrounding gelatinous substance, making it inactive. |
| Current research aims at using unsilencing techniques to potentially treat X-linked genetic disorders like Fragile X and Rett syndromes. |
| These treatments hold promise not just for females but also for males with certain mutations on the X chromosome. |
| The long-standing research efforts could lead to therapies with minimal side effects by selectively restoring function to mutated genes. |
Summary
X chromosome inactivation is a critical biological process that allows females, who have two X chromosomes, to inactivate one copy. This phenomenon is essential for balancing gene dosage between sexes and has far-reaching implications for understanding genetic disorders. The groundbreaking work by Jeannie T. Lee’s lab at Harvard Medical School unveils the complexities of how this inactivation occurs, revealing potential therapeutic avenues for conditions like Fragile X and Rett syndromes. By manipulating the processes involved in X chromosome inactivation, researchers are paving the way for innovative treatments that could benefit both females and males affected by genetic mutations on the X chromosome.